Using the following standard reduction potentials,calculate the 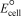 for the reaction of tin with chromium(III) hydroxide producing tin(IV) oxide and chromium under basic conditions.
for the reaction of tin with chromium(III) hydroxide producing tin(IV) oxide and chromium under basic conditions. 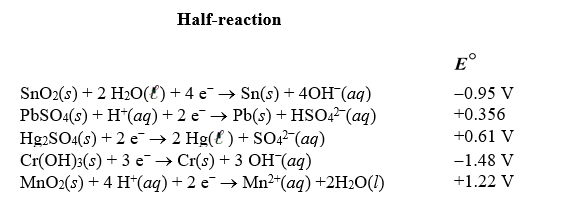
Definitions:
Stock Split
A corporate action to increase the number of outstanding shares through a proportional division, reducing the stock price accordingly.
TSX Rules
The regulations and guidelines established by the Toronto Stock Exchange (TSX) that govern trading, listing, and company compliance in Canada’s primary stock market.
Q4: Region I in the nucleotide shown is
Q6: The pH of a sample of swimming
Q17: A strong crystal field produces a
Q41: The molecular orbital description for metal bonding
Q100: In each of the following,the stronger acid
Q105: The oxidation of hydrogen by oxygen
Q122: Which of the following statements regarding the
Q132: Ethanol and dimethyl ether,which share the chemical
Q153: A phosphate buffer solution (25.00 mL sample)used
Q153: Which of the following molecules,all of