Using the following data,determine the standard cell potential 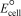 for the electrochemical cell constructed using the following reaction: Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s) . Half-reaction Standard reduction potential
for the electrochemical cell constructed using the following reaction: Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s) . Half-reaction Standard reduction potential
Zn2+(aq) + 2 e- Zn(s) -0.763
Pb2+(aq) + 2 e- Pb(s) -0.126
Definitions:
Savings Account
A bank account that earns interest and is typically used for holding money that is not intended for daily expenses.
Accumulated Amount
The total sum including the principal and the compounded interest over a period.
End-Of-Quarter Payments
Payments that are made at the conclusion of a fiscal quarter, often related to financial obligations such as loans or leases.
Annuity
A fiscal product designed to disburse a static sequence of payments to someone, chiefly utilized as a means for generating income during retirement.
Q7: Which of the following unit cells has
Q10: If half of the tetrahedral holes in
Q16: If a face-centered cubic unit cell
Q23: Which of the following is NOT a
Q29: Dissolved concentrations of copper(II)as low as 10<sup>-</sup><sup>6</sup>
Q79: Which statement about a cathode in a
Q83: What reaction occurs as sodium hydroxide
Q90: In an XRD analysis using a
Q97: The alcohol functional group has the bonding
Q125: Using the following data,determine the standard