Given the following data,calculate the approximate value of the equilibrium constant at 298 K for the reaction,Ni(NH3) 62+(aq) + 3 en(aq) Ni(en) 32+(aq) + 6 NH3(aq) ,where en represents ethylenediamine.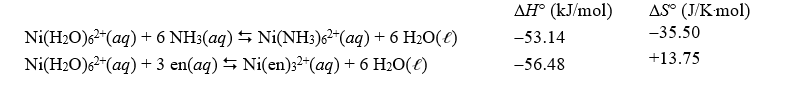
Definitions:
Vacancy Model
A vacancy model is a theoretical or practical framework used to predict or analyze the vacant positions within an organization at any given time.
Personnel Supply Requirements
This involves determining the quantity and quality of employees available in the market that meet the organization's needs.
Renewal Model
A mathematical model used in operations research and statistics to analyze the times between events in a process where each event renews the process afresh.
Probabilistic Model
A statistical model that incorporates randomness and uncertainty, and uses probabilities to predict outcomes.
Q1: Write an expression for the equilibrium constant
Q13: Use the table of standard reduction potentials
Q37: What is the chemical formula of aminetrichloroplatinate(II)?<br>A)[Pt<sub>2</sub>(NH<sub>3</sub>)Cl<sub>3</sub>]<sup>-</sup><br>B)[Pt(NH<sub>3</sub>)<sub>3</sub>Cl]<sup>-</sup><br>C)[Pt(NH<sub>3</sub>)Cl<sub>3</sub>]<sup>2</sup><sup>-</sup><br>D)[Pt(NH<sub>3</sub>)Cl<sub>3</sub>]<sup>+</sup><br>E)[Pt(NH<sub>3</sub>)Cl<sub>3</sub>]<sup>-</sup>
Q41: Which sketch best represents the qualitative molecular
Q43: Which statement does NOT correctly describe
Q57: Which of the following square planar complexes
Q96: As T approaches infinity,ln K approaches _<br>A)infinity.<br>B)zero.<br>C)
Q101: Define a conjugate acid-base pair and provide
Q111: Using the following data,determine <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6561/.jpg"
Q149: If a body-centered cubic unit cell