Phosphoric acid is a triprotic acid in water,ionizing in the following sequential steps: H3PO4 + H2O H2PO4- + H3O+ 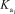 H2PO4- + H2O HPO42- + H3O+
H2PO4- + H2O HPO42- + H3O+ 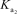 HPO42- + H2O PO43- + H3O+
HPO42- + H2O PO43- + H3O+
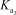 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Definitions:
File Name
A file name is the unique identifier of a file in an operating system, used to distinguish it from other files and often contains an extension indicating the file type.
Scanner Class
In Java, the Scanner class is used to read input from various sources, including user input from the console, making it useful for parsing and processing input data.
NextFloat
A method typically seen in programming languages like Java to read or generate the next floating-point number from an input source or a random number generator.
UseDelimiter
A method used in Java's Scanner class to specify a delimiter pattern, defining how text input is broken into tokens.
Q29: Magnesium oxide adopts a rock salt structure
Q29: The mechanism for the reaction 2
Q48: Consider the equilibrium CO(g)+ 2 H<sub>2</sub>(g)
Q73: For the reaction 2 N<sub>2</sub>O<sub>5</sub>(g) <span
Q92: Silver tarnish (Ag<sub>2</sub>S)can be removed by
Q96: As T approaches infinity,ln K approaches _<br>A)infinity.<br>B)zero.<br>C)
Q113: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6561/.jpg" alt=" Calculate
Q161: Indicate which of the following plots would
Q206: A mining operation needs to separate
Q221: A cup of coffee has a