Phosphoric acid is a triprotic acid in water,ionizing in the following sequential steps: H3PO4 + H2O H2PO4- + H3O+ 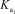 = 7.11 *10-3
= 7.11 *10-3
H2PO4- + H2O HPO42- + H3O+
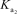 = 6.32 *10-8
= 6.32 *10-8
HPO42- + H2O PO43- + H3O+
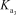 = 4.5 *10-13
= 4.5 *10-13
What is the pH of a 0.20 M Na3PO4 solution?
Definitions:
Standard Cost System
An accounting system that uses cost estimates for materials, labor, and overhead to assess operational efficiency and inventory valuation.
Reportable Segment
A part of a company that can be identified for reporting purposes based on its products, services, or geographical location.
General Corporate Assets
Assets owned by a corporation that are used in the overall operations and support of the business, including both tangible and intangible assets.
Research and Development
Activities undertaken by a company to innovate and introduce new products or services, often categorized as an expense.
Q11: Calculate the equilibrium constant for the
Q13: Smog created by the interaction of sunlight
Q53: The energy supplied by a battery can
Q84: Graphite and diamond are examples of _
Q90: For the second-order reaction,NO(g)+ F<sub>2</sub>(g) <span
Q100: The linear form of the Arrhenius equation
Q104: The following reaction occurs in acidic
Q107: The structure of the acetylacetonate ligand is
Q162: If in using a lead-acid battery to
Q164: In an elementary step of a reaction