There are many reactions involving nitrogen,oxygen,and nitrogen oxides at high temperatures.At 1000.0 K,the following reactions have the given equilibrium constants: N2O4(g) 2 NO2(g) K1 = 1.5 106 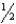 N2(g) + O2(g) NO2(g) K2 = 1.2 10-5
N2(g) + O2(g) NO2(g) K2 = 1.2 10-5
Calculate the theoretical value of the equilibrium constant for N2(g) + 2 O2(g) N2O4(g) .
Definitions:
External Adaptation
The process by which an organization adjusts and changes its strategies and operations in response to external environmental factors.
Internal Differentiation
The division of roles, responsibilities, and tasks within an organization or group to specialize and increase efficiency.
Cultural Symbols
Objects, signs, or emblems that represent specific ideas, values, or traditions within a culture.
Shared Behaviors
Actions or practices that are commonly exhibited by members of a group, organization, or community.
Q23: Phosphoric acid is a triprotic acid
Q40: What is the coordination number of the
Q48: For the reaction 2 A +
Q71: Which of the following is the K<sub>a</sub>
Q82: Which statement,A-D,is NOT correct? If all
Q102: The change in standard molar entropy,
Q143: NO gas is converted to NO<sub>2</sub>
Q152: What is the oxidation state of nickel
Q153: Chromium often is electroplated on other metals
Q183: For the reaction 2 A +