The values of 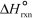 and
and 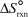 are shown for the following equilibrium reaction.What makes the equilibrium constant larger at low temperature? 2 NO2(g) N2O4(g) H = -58.02 kJ/mol, S =-176.5 J/mol . K
are shown for the following equilibrium reaction.What makes the equilibrium constant larger at low temperature? 2 NO2(g) N2O4(g) H = -58.02 kJ/mol, S =-176.5 J/mol . K
Definitions:
Real NNP
Net National Product adjusted for inflation; measures the value of goods and services produced by a country's economy, less the value of goods and services used up in production, adjusted for price changes.
Price Index
A statistical measurement that tracks changes in the price level of a basket of goods and services over time.
Inventories
The raw materials, work-in-process products, and finished goods that are considered to be the portion of a business's assets that are ready or will be ready for sale.
GDP
The total value of all end products and services created within a country's borders during a certain time frame defines Gross Domestic Product.
Q22: Which of the following processes is predicted
Q26: What is the osmotic pressure of
Q62: Magnesium chloride is often used to
Q76: Which of the following statements regarding reaction
Q80: Consider the equilibrium,2 NOCl(g) <span class="ql-formula"
Q91: For the equilibrium (CH<sub>3</sub>)<sub>3</sub>CCl(g) <span class="ql-formula"
Q119: Of the three modes of molecular motion-vibration,rotation,and
Q128: Considering the tabulated values for the thermodynamic
Q135: What is the correct formula for sodium
Q208: The rate constant for the reaction