The slope and intercept of an Arrhenius plot made for the first-order decomposition reaction, H2O2 H2O + 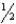 O2,are -12,500 K and 33.02,respectively.What is the value of k at 303 K?
O2,are -12,500 K and 33.02,respectively.What is the value of k at 303 K?
Definitions:
Donee Beneficiary
In contract law, a third party who benefits from a contract between two other parties, particularly when the benefit was intended as a gift.
Promisee
The person to whom a promise or commitment is made, especially in the context of a contractual agreement.
Promisor
An individual or entity that makes a promise or agreement to perform a specified action in a contract.
Contractual Rights
The specific entitlements and obligations that are established within a contract, allowing the parties involved to enforce the terms legally.
Q2: A sample of gas at 475.0<sup>
Q15: The solubility product for Al(OH)<sub>3</sub> is written
Q70: Based on lattice energies,which of the following
Q75: What is the boiling point of
Q87: When one mole of solid ammonia
Q98: The reaction of NO gas with
Q147: Which combination of solutions is the best
Q168: Which one of the following salts forms
Q182: Which one of the following is a
Q188: Given the following data for the