You Wish to Prepare a Solution of Methanol (CH3OH,32 CCalculate the Mole Fraction of Ethanol in the Solution That
You wish to prepare a solution of methanol (CH3OH,32.04 g/mol) and ethanol (CH3CH2OH,46.07 g/mol) that has a total vapor pressure of 66 torr at 20 C.Calculate the mole fraction of ethanol in the solution that will produce the desired pressure. 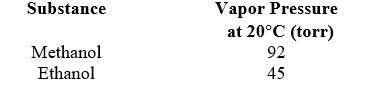
Definitions:
Cum Dividend
A term indicating that a stock is trading with the right to receive the most recently declared dividend.
Shareholders of Record
Shareholders of record are individuals or entities that are officially recorded by a company as the legal owners of its shares on a specific date.
September
The ninth month of the year in the Gregorian calendar.
Positive Net Present Value
An indicator that an investment is expected to generate more cash than the initial amount invested, viewed positively in capital budgeting.
Q7: N<sub>2</sub>O (laughing gas)is used as an anesthetic.A
Q27: A proposed mechanism for the photodecomposition
Q55: The molar entropies of carbon monoxide
Q85: Given the following data,determine the rate
Q101: The aroma from almonds and cherries is
Q105: Ammonium nitrate (80.04 g/mol)decomposes at high
Q120: The energy stored in chemical bonds is
Q154: What is the root-mean-square speed of a
Q166: Ammonia gas (NH<sub>3</sub>)is produced from hydrogen
Q207: Given the following data for the