Ammonium nitrate (80.04 g/mol)decomposes at high temperatures to form nitrogen,oxygen,and water vapor.Suppose 750 grams decomposes at 350°C and 0.98 atm,generating gases that expand by about 960 L.How much P-V work is done? What is the approximate change in internal energy ( E )of the reaction system? (1 L . atm = 101.3 J)
NH4NO3(s) N2(g)+ 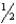 O2(g)+ H2O(g) H =-118 kJ/mol
O2(g)+ H2O(g) H =-118 kJ/mol
Definitions:
Free Menial Labor
Work, often unskilled and poorly paid, performed without remuneration or for minimal compensation.
Careers
The progression of work-related activities and experiences across an individual's lifetime, which can include jobs, education, and volunteer work.
Transnational Corporation
A large company that operates in multiple countries, extending its services and products beyond its home country's borders.
World of Work
Refers to the realm of employment and labor markets, including job opportunities, employment trends, and workplace practices.
Q5: When 10.5 g KBr(s)dissolves in 125 g
Q8: In an experiment,138 g of graphite is
Q81: A 15 g piece of iron [C<sub>p</sub>
Q112: Isopropyl alcohol has a boiling point
Q124: Which of the following statements regarding calorimetry
Q140: Which of the following statements regarding water
Q147: Most chloride salts are soluble.Which of the
Q150: The drinking water standard of the
Q188: Use the bond energies in the table
Q208: In which one of the following compounds