Identify the hybridization of atomic orbitals for atoms N1,N2,C1,C2,and C3 in caffeine,which is shown below.Explain why you think this molecule is planar or nonplanar. 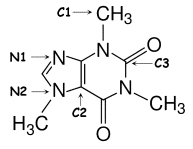
Definitions:
Groupthink
An occurrence within a group where the need for agreement or adherence leads to illogical or ineffectual choices.
Deindividuation
A psychological state where an individual loses self-awareness and a sense of individuality, often leading to disinhibited behavior, usually in group settings.
Deindividuation
A psychological state where an individual loses self-awareness and self-restraint, often occurring in group settings that foster anonymity.
Social Facilitation
When the mere presence of others improves performance.
Q31: Which liquid,water or ethanol,would you expect to
Q35: Copper metal reacts with nitric acid to
Q38: What is the formal charge of the
Q39: Describe the similarity and the difference between
Q99: Which one of the following items does
Q99: Arrange the following elements in order of
Q104: The relative energies (strengths)of the intermolecular forces
Q116: Paris green is a highly toxic emerald
Q121: What is the symbol for sulfur?<br>A)Si<br>B)Sc<br>C)Su<br>D)S<br>E)Sf
Q139: How many shared electron pairs are there