The energy change for an electronic transition in a one-electron atom or ion (H,He+,Li2+,etc.) from ninitial to nfinal is given by 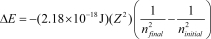 ,where Z is the atomic number. How much energy is required to ionize a ground-state He+ ion (nfinal = ) ?
,where Z is the atomic number. How much energy is required to ionize a ground-state He+ ion (nfinal = ) ?
Definitions:
Begging the Question
A logical fallacy where the conclusion of an argument is assumed in the premise, offering no real evidence.
Society
A community of individuals living together and interacting with each other.
Circular Argument
An argumentative fallacy where the conclusion is also one of its premises.
Accident
An unintended or unforeseeable event causing damage or injury.
Q3: If the speed of an object triples,its
Q11: You create a new and unique ionic
Q15: The energy change for an electronic transition
Q28: The work function of sodium is
Q59: When sulfur-containing fuels are burned,sulfur trioxide (SO<sub>3</sub>)is
Q113: The densities of ethylene glycol and of
Q134: The potential energy associated with gravity can
Q134: If the principal quantum number is seven
Q136: Not all the bond vibrations of carbon
Q150: Which of the following has a central