Consider the reaction H2(g) + 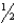 O2(g) H2O(l) H° = -286 kJ
O2(g) H2O(l) H° = -286 kJ
Which of the following is true?
Definitions:
Operating Activities
Operating activities involve the core business functions of a company, including production, distribution, and sales, determining cash flow from primary business operations.
Interest Expense
The monetary burden placed on an entity due to borrowing capital over a specified timeframe.
Market Rate
The prevailing interest rate available in the market for loans or the return on investment securities.
Adjusting Entry
An accounting journal entry made at the end of an accounting period to update the accounts and ensure they comply with the accrual basis of accounting.
Q14: Which form of electromagnetic radiation has the
Q28: A chemical reaction has the equation:
Q47: The bag is emptied and refilled,successively,with gases
Q49: For which gas are the molecules diatomic?<br>A)He<br>B)Cl<sub>2</sub><br>C)CH<sub>4</sub><br>D)NH<sub>3</sub><br>E)all
Q57: How many possible values are there for
Q86: Consider the following Lewis structure: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg"
Q93: How many grams of NaOH are
Q102: Phosphoric acid can be prepared by
Q120: In the hydrogen spectrum,what is the
Q127: Cyclobutane has 109° bond angles like all