Oxygen gas,generated by the reaction 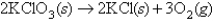 is collected over water at 27°C in a 1.55-L vessel at a total pressure of 1.00 atm.(The vapor pressure of H2O at 27°C is 26.0 torr. ) How many moles of KClO3 were consumed in the reaction?
is collected over water at 27°C in a 1.55-L vessel at a total pressure of 1.00 atm.(The vapor pressure of H2O at 27°C is 26.0 torr. ) How many moles of KClO3 were consumed in the reaction?
Definitions:
Dichotomous Thinking
A cognitive distortion involving viewing things in black and white categories, without recognizing any middle ground.
Rational Grounding
A cognitive behavioral technique that involves using reason and rational thinking to challenge and overcome irrational beliefs and fears.
Self-Talk
The internal dialogue that consists of the thoughts and beliefs an individual has about themselves and their experiences.
Irrational Belief Systems
Deeply held but unreasonable convictions or assumptions that can lead to emotional and behavioral issues.
Q4: The bond angles about the carbon atom
Q18: An example of a secondary structure of
Q40: The following equation describes the oxidation of
Q50: Calculate the work associated with the
Q66: How many electrons are shared between carbons
Q86: A 2.80-g sample of an oxide of
Q88: Which one of the following statements
Q93: Which of the following are incorrectly
Q114: The calcium atom is much larger than
Q114: The building blocks of all proteins are<br>A)pleated