One of the hopes for solving the world's energy problem is to make use of the fusion reaction: 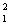 H +
H + 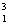 H
H 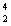 He +
He + 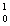 n + energy How much energy is released when one mole of deuterium is fused with one mole of tritium according to the above reaction? The masses of the atoms and the neutrons are:
n + energy How much energy is released when one mole of deuterium is fused with one mole of tritium according to the above reaction? The masses of the atoms and the neutrons are: 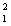 H = 2.0140 amu
H = 2.0140 amu 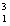 H = 3.01605 amu
H = 3.01605 amu 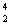 He = 4.002603 amu
He = 4.002603 amu 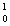 n = 1.008665 amu
n = 1.008665 amu
The speed of light is 2.9979 108 m/s
Definitions:
Recommendations
Suggestions or proposals as to the best course of action, especially one put forward by someone with expertise or authority.
External
Pertaining to factors, influences, or conditions that exist or originate outside of an organization or system.
Unsolicited Proposal
A proposal submitted to an organization without their request, often aiming to solve a problem or offer services.
Attention-Getter
An element or strategy used to capture the audience's attention at the beginning of a communication.
Q15: The half-life of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6423/.jpg" alt="The half-life
Q21: If one mole of oxygen-16 were formed
Q28: Choose the most likely pattern for the
Q29: How many oxygen atoms are there in
Q60: Which type of radiation has the lowest
Q79: Use the following data to determine the
Q85: What is the pH at point I
Q98: When heat is added to proteins,the
Q129: Choose the group that matches the following
Q157: All of the following statements about carbohydrates