In order to determine the identity of a particular lanthanide metal Sm,a voltaic cell is constructed at 25°C with the anode consisting of the lanthanide metal as the electrode immersed in a solution of 0.0819 M SmCl3,and the cathode consisting of a copper electrode immersed in a 1.00 M Cu(NO3) 2 solution.The two half-reactions are as follows: Sm(s) Sm3+(aq) + 3e-
Cu2+(aq) + 2e- Cu(s)
The potential measured across the cell is 2.67 V.What is the identity of the metal?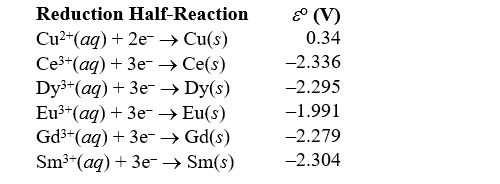
Definitions:
Q16: When the Pd-106 nucleus is struck with
Q25: In which of the following cases
Q35: Silver acetate,AgC<sub>2</sub>H<sub>3</sub>O<sub>2,</sub> is a sparingly soluble
Q48: The following question refers to a
Q64: Find the equilibrium concentration of Mn(C<sub>2</sub>O<sub>4</sub>)in
Q68: The decomposition of nitric acid in sunlight
Q76: A certain complex ion has a distorted
Q79: After 50.0 mL of 0.20 M NaOH
Q106: For a spontaneous exothermic process,which of
Q129: Choose the group that matches the following