Consider the galvanic cell shown below (the contents of each half-cell are written beneath each compartment) : 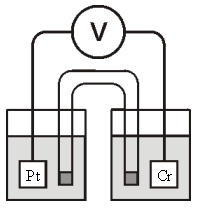 0.50 M Br2 0.20 M Cr3+
0.50 M Br2 0.20 M Cr3+
0.10 M Br-
The standard reduction potentials are as follows:
Cr3+(aq) + 3e- Cr(s)
° = -0.733 V
Br2(aq) + 2e- 2Br-(aq)
° = +1.090 V
What is the value of for this cell at 25°C?
Definitions:
Patient-to-Nurse Ratio
The number of patients assigned to a nurse during a shift, affecting workload and the quality of care provided.
Wound Care
The management and treatment of wounds, including cleaning, disinfecting, and promoting healing to prevent infection and complications.
Quality Improvement
An ongoing process of evaluating and enhancing the quality of services or products, aimed at achieving superior outcomes in healthcare or other sectors.
Status of Nursing
The current state or condition of the nursing profession, reflecting on its challenges, advancements, and societal perception.
Q35: Calculate the pH of a 0.59
Q41: Which of the following balanced equations
Q47: A concentration cell is constructed with
Q47: Solid calcium hydroxide is dissolved in
Q54: Calculate <span class="ql-formula" data-value="\Delta"><span
Q55: Which unit takes into account the relative
Q58: For the process Co(NH<sub>3</sub>)<sub>5</sub>Cl<sup>2+</sup> + Cl<sup>-</sup>
Q61: The heat of vaporization for 1.0
Q77: Which of the following statements is/are
Q147: The elements in this group are termed