A solution contains 0.018 moles each of I-,Br-,and Cl-.When the solution is mixed with 200 mL of 0.24 M AgNO3,how much AgCl(s) precipitates out? 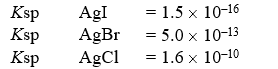
Definitions:
S Corporations
A type of corporation that meets specific Internal Revenue Code requirements, allowing profits to be passed directly to shareholders and avoid double taxation.
Q7: Which statement is always true of the
Q16: Given the following values of equilibrium
Q39: Calculate the pH at the equivalence point
Q52: The conjugate acid and conjugate base of
Q53: Substance X has a heat of
Q58: How do the equilibrium concentrations of the
Q59: At a particular temperature,N<sub>2</sub>O<sub>5</sub> decomposes according
Q66: Balance the following equation: MnO<sub>4</sub><sup>-</sup> +
Q71: The standard potential for the reaction
Q118: A solution in which the pOH is