What is the maximum concentration of iodide ions that will precipitate AgI but not PbI2 from a solution that is 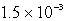 M each in Ag+ and Pb2+? For AgI,Ksp = 1.5 10-16 and for PbI2,Ksp = 1.4 10-8.
M each in Ag+ and Pb2+? For AgI,Ksp = 1.5 10-16 and for PbI2,Ksp = 1.4 10-8.
Definitions:
Direct-Mail Marketer
A marketing professional or company that specializes in sending promotional materials directly to potential customers through postal or electronic mail.
Quick-Response Retailer
Retailers that use efficient supply chains and data analysis to quickly adjust to market trends and consumer demands.
Home Delivery Retailer
A business that sells products directly to customers and delivers them to their homes.
Online Shopping
The act of purchasing goods or services over the internet, involving the search, selection, and payment of products via electronic platforms.
Q7: The following data were obtained for
Q7: How do the pHs of the buffered
Q19: In which direction do electrons flow in
Q19: Assume that the enthalpy of fusion of
Q21: Which of the following statements is true?<br>A)Ions
Q43: Calculate the pH of a solution
Q54: Calculate <span class="ql-formula" data-value="\Delta"><span
Q59: When the following reaction is balanced
Q97: For a reaction in a voltaic
Q114: <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mi mathvariant="normal">Δ</mi></mrow><annotation