A solution contains 0.018 moles each of I-,Br-,and Cl-.When the solution is mixed with 200 mL of 0.24 M AgNO3,how much AgCl(s) precipitates out? 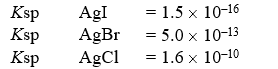
Definitions:
Transformational Leadership
A leadership style where leaders inspire and motivate employees to exceed their own interests for the sake of the organization and encourage innovation.
Statutory Lien
A legal claim on property, either personal or real, granted by law to creditors as security for a debt or obligation.
Debtor
An individual or entity that owes money or is in debt to another party.
Composition Agreement
An arrangement between a debtor and their creditors in which the creditors agree to accept a partial payment in satisfaction of the debts owed.
Q11: Which statement is true?<br>A)All real processes are
Q47: The first people to attempt to explain
Q49: Determine <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q55: Which unit takes into account the relative
Q65: Which of the following pairs is incorrect?<br>A)iodine
Q89: Consider a galvanic cell with a
Q93: What is the limiting reagent in the
Q95: The equilibrium constant K for the
Q98: The following question refers to a
Q140: Which of the following is the