For the reaction NO(g) + 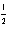 O2(g)
O2(g)  NO2(g) at 750°C,the equilibrium constant Kc equals:
NO2(g) at 750°C,the equilibrium constant Kc equals:
Definitions:
Goodness Of Fit Model
A concept in developmental psychology that explains how well an individual's temperament matches with their environment, affecting their development and behavior.
Learning Styles
The preferential way in which an individual absorbs, processes, comprehends and retains information.
Temperament
Inherent personality traits and tendencies that influence how individuals react to their environment, often observable from a young age.
Academic Standards
The set criteria or benchmarks for academic performance and achievement within educational institutions.
Q5: The K<sub>sp</sub> for Mn(OH)<sub>2</sub> is 2.0
Q7: You are asked to determine the perimeter
Q11: Solid KF has a lattice energy of
Q16: Given the following values of equilibrium
Q25: A solution of 8.01 M formic
Q35: Calculate the pH of a 0.59
Q35: Consider the titration of 300.0 mL
Q47: Which of the following is most likely
Q49: You have a 250.0-mL sample of
Q80: In a 0.1 molar solution of NaCl