Different concentrations of ions on either side of a cell membrane constitute a concentration cell, which is described by the Nernst equation given below. A cell must expend energy to maintain this concentration gradient by moving ions from one side of the membrane to the other. How much energy must be expended to transport 1 mole of sodium ions across a membrane when the sodium ion concentration is 0.10 M on one side of the membrane and 0.010 M on the other side, and the temperature is 37C (body temperature) ? (Given: 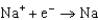 ,
, 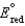 2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K) )
2.71 V; F 96,485 C; 1 J 1 C V; R 8.315 J / (mol K) )
E E - 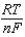 InQ
InQ
Definitions:
Workplace Harassment
Unwelcome conduct in a professional environment that can lead to a hostile work atmosphere or affect an employee's job performance.
Outside the Scope
Referring to matters that fall beyond the range of authority, interest, or area of consideration designated for a particular discussion, activity, or project.
Vicariously Liable
Vicariously liable means being held legally responsible for the actions of another person, such as an employer being liable for acts of their employees.
Inherently Dangerous
Activities or objects that carry a risk of harm or damage as a natural part of their existence or function.
Q4: Refer to the drawings in Figure 10.2
Q11: Which of these individuals is likely to
Q17: Which of the following is the best
Q29: Which of the following traits is an
Q32: You have isolated DNA from three different
Q33: Radon-222 has a half-life of 3.8 days.
Q59: The relationship between genes S and N
Q77: Draw all of the isomers for butanol.
Q140: What monomer is produced when starch is
Q142: How many unpaired electrons are there in