Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has chromium, Cr, and cadmium, Cd, electrodes? Also, identify the anode. Standard Reduction
Potentials (volts) in Aqueous Solution 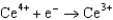 1.70
1.70 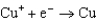 0.520
0.520 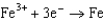 0.036
0.036 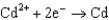 0.400
0.400 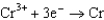 0.73
0.73 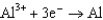 1.66
1.66
Definitions:
Operating Activities
Business actions that are involved in the day-to-day functions of producing goods, offering services, and other core operations.
Direct Method
A way of allocating service department costs directly to production departments without any consideration for services rendered between service departments.
Operating Activities
Activities that relate directly to the operation of the company, including production, sales, and day-to-day administrative functions.
Net Sales Adjusted
The revenue a company generates from its primary operations after deducting returns, allowances, and discounts.
Q23: In an experiment, 1.00 atm of N<sub>2</sub>(g)
Q52: It is claimed that iron-56 is the
Q54: Which of the following species is least
Q94: Radiation seed therapy is a common method
Q98: At what temperature does the Fe(s) <img
Q100: Among other problems with smoking, there is
Q118: A solution of nitrous acid (0.13 M,
Q118: Which type of radiation has the greatest
Q119: Oleic acid, shown below, is _ <img
Q141: Write appropriate chemical reaction equations to show