Use the table of standard reduction potentials below to identify the metal or metal ion that is the weakest reducing agent. Standard Reduction
Potentials (volts) in Aqueous Solution 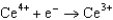 1.70
1.70 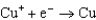 0.520
0.520  0.036
0.036 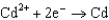 0.400
0.400 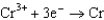 0.73
0.73 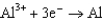 1.66
1.66
Definitions:
Homosexual Men
Male individuals who experience sexual attraction to other males.
Estrogen
A group of steroid hormones that play a crucial role in the development and regulation of the female reproductive system and secondary sexual characteristics.
Anterior Hypothalamus
A region at the front of the hypothalamus involved in regulating temperature, reproductive behaviors, and sleep.
Neurons
Specialized cells within the nervous system that transmit information to other neurons, muscle cells, or gland cells.
Q5: The peak in nuclear binding energy/nucleon occurs
Q49: During a spontaneous chemical reaction, it is
Q62: How many chiral centers are there in
Q64: The most suitable acid-base indicator for a
Q82: If the free-energy change of the following
Q108: The oxidation of methanol, as described by
Q108: The ordering of subunits, such as amino
Q128: Determine <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine
Q135: How many unpaired electrons are there in
Q138: Radioactive decay is a _-order process.<br>A)Zero<br>B)First<br>C)Second<br>D)zero-or first<br>E)zero-first-or