Consider the following standard reduction potentials. Reduction Half-Reaction 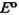 (volts)
(volts) 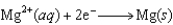 2.38
2.38 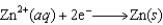 0.76
0.76 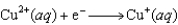 0.16
0.16
The Mg/Mg2 half-reaction can be paired with the other two to produce voltaic cells because ________
Definitions:
ANOVA Model
Short for Analysis of Variance, a statistical model used to compare the means of three or more samples to determine if at least one of the sample means significantly differs from the others.
Degrees of Freedom
The number of independent values or quantities which can be assigned to a statistical distribution without violating any constraints.
Flake Size
Flake size refers to the dimensions of individual flakes within a material, often critical in determining the material's properties and applications.
Tree Species
Types or kinds of trees, characterized by distinct genetic traits or botanical classifications.
Q4: The equilibrium constant for a given reaction
Q25: Consider the following standard reduction potentials. Reduction
Q27: Glycolic acid, which is a monoprotic acid
Q36: The solubility of PbI<sub>2</sub> is 0.756 g
Q38: Which process converts a proton into a
Q38: The work involved in moving exactly 1
Q56: The ion found in chlorophyll is _<br>A)Zn<sup>2</sup><font
Q70: Ion channels in cell membranes control selective
Q102: Lead pipes were used at one time
Q146: Silver tarnishes due to the formation of