Consider the following standard reduction potentials. Reduction Half-Reaction 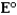 (volts)
(volts) 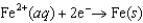 0.45
0.45 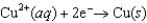 0.34
0.34 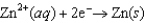 0.76
0.76 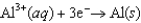 1.66
1.66 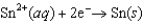 0.14
0.14
Which corrosion reaction when two metals come in contact is not likely to occur?
Definitions:
Production
The process of creating, manufacturing, or improving goods and services.
R&D
Research and Development, the phase in a business or project aimed at discovering new knowledge that might lead to new products, processes, or services.
Integrator Role
A function in organizations and teams where the individual is responsible for bringing together different elements, information, and resources for cohesive operation and strategy.
Liaison Role
A role that involves facilitating coordination and communication between different parts of an organization or between organizations.
Q5: The cation BiO<font face="symbol"><sup></sup></font> is found in
Q25: What repulsive forces must be overcome for
Q84: Copper metal is purified by electrolysis. How
Q88: A derivative of glycopyrollate, a medication often
Q94: What is the K<sub>a</sub> for nitrous acid,
Q113: In its linear form, which functional groups
Q126: Use the table of standard reduction potentials
Q134: When a positron and an electron collide,
Q136: The standard molar entropy of lead(II) bromide
Q164: For a particular hypothetical reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"