Which statement about the reaction below, where en ethylenediamine (H2NCH2CH2NH2) , is not correct? 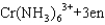
Definitions:
Fiscal Policy
Government policies related to taxation and spending that aim to influence the economy, manage inflation, and stimulate economic growth.
Restrictive Fiscal Policy
Fiscal measures implemented by a government to reduce its spending and/or increase taxes with the aim of slowing down an overheating economy.
Economic Plunge
A sudden and sharp decline in economic activity, often characterized by a decrease in GDP, investment, and employment.
Tax Rates
The percentages at which income, property, and sales are taxed by governments.
Q13: Calculate the K<sub>sp</sub> for Li<sub>3</sub>PO<sub>4 </sub>if at
Q23: If the potential of a voltaic cell
Q37: Given the following reactions and associated equilibrium
Q51: What is the pH of a 0.055
Q78: An electrochemical cell has both a silver
Q90: Which of the following figures illustrates best
Q121: Let s be the number of moles
Q122: The natural abundance of uranium-235 is _<br>A)a
Q125: Indicate which of the following has the
Q132: Which graph below describes the decay of