The chemical equilibrium constant for the following reaction is 14.5. CH3OH(g)  CO(g) 2H2(g)
CO(g) 2H2(g)
What is the value of the equilibrium constant for the following reaction? 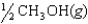
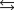
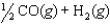
Definitions:
Risk For Cancer
The probability or likelihood that an individual will develop cancer over a certain period of time, considering various factors.
Incidence
The occurrence, rate, or frequency of a disease, crime, or something undesirable within a given population over a specific period of time.
Mortality
The occurrence of death within a population; often used in statistics to quantify the ratio of deaths in a particular population during a specific time frame.
Breast And Prostate Cancer
Types of cancer, with breast cancer occurring in the breast tissue and prostate cancer originating in the prostate gland, both with various treatment options and outcomes.
Q9: Which of the following shows a small
Q10: Pure water at any temperature has _<br>A)a
Q21: Which of the following is a colligative
Q75: Which of the following statements must be
Q100: Cylinders of NO gas may contain small
Q102: Write an expression for the equilibrium constant
Q109: What is the value of the equilibrium
Q111: In antifluorite structures, _<br>A)cations are smaller than
Q130: Jane can accept that <font face="symbol"></font>G<font face="symbol"></font>
Q132: Which of the following ionic compounds would