The sulfide ion, S2, reacts with water as a weak base:
S2 H2O 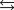 SH OH K 1.0
SH OH K 1.0
If sodium sulfide were dissolved in water to make a solution of 0.50 M, what would be the resulting concentration of OH?
Definitions:
Debtors
Individuals or entities that owe money to others; they are the borrowers in financial transactions.
Computer Programmer
A computer programmer is a professional skilled in writing, testing, and maintaining code that enables software and applications to function.
Psychologically
Pertaining to the study of the mind and behavior, or relating to mental processes and emotional responses.
Unemployment Rate
The proportion of people in the workforce who are unemployed and seeking work actively.
Q31: What is the molar concentration of Ag<font
Q38: Which of the following compounds is capable
Q46: The energy profiles for four different reactions
Q55: Which molecule has the largest dipole?<br>A)methane (CH<sub>4</sub>)<br>B)ammonia
Q58: Aluminum alloys are more desirable than steel
Q69: Suppose <font face="symbol"></font>G<font face="symbol"></font> is 25.0 kJ/mol
Q97: A petroleum company separates benzene (78 g/mol)
Q112: The reaction 2NO(g) <font face="symbol"></font> O<sub>2</sub>(g) <img
Q129: Determine <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine <font
Q144: In a catalyzed reaction, the activation energy