Water can decompose at an elevated temperature to give hydrogen and oxygen according to the equation below. At a particular temperature in a vessel containing water vapor and air, the partial pressures of H2O, H2, and O2 are 0.055 atm, 0.0065 atm, and 0.19 atm, respectively. What is the value of the reaction quotient, QP, for this reaction under these conditions? 2H2O(g) 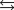 2H2(g) O2(g)
2H2(g) O2(g)
Definitions:
Fair-Return Price
A price that covers production costs and allows for a normal profit margin, considered equitable for both producers and consumers.
Regulating Monopolies
Government policies or laws designed to control or limit the power of monopoly firms to ensure fair competition and protect consumers.
Price Discrimination
The strategy of selling the same product or service at different prices to different customers, often based on the willingness to pay, market segment, or purchase volume.
Conditions
The circumstances or criteria affecting the operation of markets or the status of economic factors.
Q12: Which statement A-D does not describe a
Q23: Vitamin C, which is ascorbic acid, is
Q25: For an alloy composed of two elements
Q28: For the rate law Rate <font face="symbol"></font>
Q42: Phosphoric acid is a triprotic acid, ionizing
Q55: What is the most important use for
Q108: Increasing the temperature of an exothermic reaction
Q124: Which graph best describes how the vapor
Q143: A solution is prepared by adding 0.250
Q149: For the reaction 2A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="For