The equilibrium constant for the formation of hydrogen iodide from hydrogen and iodine is 45.0 at a certain temperature. H2(g) I2(s) 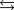 2 HI(g)
2 HI(g)
Which of the following is true regarding this equilibrium?
I.The reaction is product-favored.
II.The reaction is reactant-favored.
III.Equilibrium lies to the right.
IV.Equilibrium lies to the left.
Definitions:
Deterritorialization
A complex concept often used in social sciences to describe the loss of the cultural, social, or geographical ties that define a place or a community.
Interrelated Flows
Processes or movements that are interconnected, influencing and interacting with each other in a system or network.
Hybridization
The process or act of combining or blending two different elements, such as cultures, technologies, or species, to produce something new or more effective.
Glocalization
A strategy that combines global approaches to local markets by adapting products or services to fit local cultures and preferences.
Q20: Electrochemical cell potentials can be used to
Q47: In a two-component alloy the more abundant
Q51: What is the solubility of barium sulfate
Q53: Identify the weakest and strongest acids in
Q63: The equilibrium constant for the formation of
Q90: In a simple equilibrium A <font face="symbol"></font>
Q93: NO<sub>2</sub> contributes to the "brown haze" associated
Q121: Coulomb's law states that the interaction energy
Q129: Determine <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Determine <font
Q132: Noxious NO gas can form from N<sub>2</sub>