Water can decompose at an elevated temperature to give hydrogen and oxygen according to the equation below. At a particular temperature in a vessel containing water vapor and air, the partial pressures of H2O, H2, and O2 are 0.055 atm, 0.0065 atm, and 0.19 atm, respectively. What is the value of the reaction quotient, QP, for this reaction under these conditions? 2H2O(g) 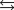 2H2(g) O2(g)
2H2(g) O2(g)
Definitions:
Contact
The act of communication or interaction between individuals, or the information needed to initiate such communication.
Topic Sentence
A sentence that expresses the main idea of a paragraph or section of a text.
Business Report
A formal document that presents analysis, research, or information on business-related subjects, aimed at supporting decision-making processes.
Mandatory
Required by law or rules; obligatory.
Q3: Which expression corresponds to the equilibrium constant
Q6: The concentration of acetic acid in vinegar
Q14: The equilibrium constants for the two reactions
Q76: A reaction with a low enthalpy of
Q81: Which of the following statements is/are correct?
Q85: Given the following data relevant to the
Q86: The energy profiles for four different reactions
Q88: Aqueous solutions of _ are basic.<br>A)NaF<br>B)NaCl<br>C)NaBr<br>D)NH<sub>4</sub>I<br>E)KI
Q123: A solution is prepared by adding 0.250
Q174: Heat transfer from the system to the