Carbon dioxide and argon, which is a noble or inert gas, are placed in a vessel and heated. Equilibrium is reached at some particular temperature. Carbon dioxide dissociates by the following reaction. 2CO2(g) 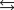 2CO(g) O2(g)
2CO(g) O2(g)
What is the result of increasing the pressure by adding more argon?
I. The equilibrium shifts to produce more carbon dioxide and reduce the pressure.
II. The equilibrium shifts to remove carbon dioxide and reduce the pressure.
III. The equilibrium does not change because argon is not involved in the reaction.
IV. The equilibrium does not change because the concentrations are not affected.
Definitions:
Least Squares Regression Line
A statistical method used to find a straight line that best fits a set of data points by minimizing the sum of the squares of the vertical distances of the points from the line.
Income
The amount of money received by a person or organization, often from employment, investments, or business ventures.
Education
The act of obtaining or providing structured learning, particularly in educational institutions like schools or universities.
Least Squares Regression Line
A straight line that best fits the data points in a scatterplot, minimizing the sum of the squares of the vertical distances of the points from the line.
Q5: Determine the entropy change for the reaction
Q5: Given the following data for the reaction
Q17: Polyethylene terephthalate, or Dacron, is a polymer
Q24: The freezing point of a 0.0925 m
Q61: An increase in the number of moles
Q80: Identify the Lewis acid in the following
Q86: Write out the five steps of the
Q93: In ionic solids, one ion often occupies
Q96: For an equilibrium reaction with K <font
Q100: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>