The reaction of nitrogen gas with oxygen gas, shown here, has a Kp value of 0.25. If a closed vessel was charged with the two reactants, each at an initial partial pressure of 0.250 atm, what would be the equilibrium partial pressure of NO(g) ? N2(g) O2(g) 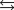 2NO(g)
2NO(g)
Definitions:
Government Regulation
Government Regulation involves the imposition of rules by government authorities to control or manage market activities, with the aim of protecting public interest.
Creative Destruction
is a concept in economics where innovation radically transforms or destroys existing industries or ways of doing business, leading to new industries and opportunities.
Widespread Costs
Expenses that impact a large sector of the population or numerous aspects of an economy, often relating to public policies or externalities.
Benefits Accrue
The accumulation of advantages or positive results over time from an action or investment.
Q7: What are the characteristics of a pH
Q15: Which of the following is true regarding
Q24: A proposed mechanism for the reduction of
Q31: Which of the following compounds will have
Q58: Which of the following compounds contains a
Q91: Lactic acid, which is found in milk
Q102: Lead pipes were used at one time
Q117: A 376 mg sample of a nonelectrolyte
Q131: Calculate the lattice energy of magnesium chloride
Q142: Boltzmann derived the relationship S <font face="symbol"></font>