Calculate the lattice energy of magnesium chloride from the following data: First ionization energy of Mg: 738 kJ/mol
Second ionization energy of Mg: 1,451 kJ/mol
Electron affinity of Cl: 349 kJ/mol
Energy to vaporize Mg: 147.1 kJ/mol
Cl2 bond energy: 243 kJ/mol
Energy change for the reaction: 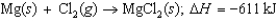
Definitions:
Power Assertion
One method parents use to enforce control of children’s behavior. Can involve physical punishment or the threat of physical punishment, or it can involve withdrawal of privileges. Effective for the immediate control of behavior, but over the long term, children show higher levels of anger, aggressiveness and anxiety.
Gentle Discipline
A parenting and educational approach that uses positive reinforcement and understanding rather than punishment to guide behavior.
Compliance
The act of conforming to or following rules, regulations, or standards set by others.
Concept of Self
An individual's perception of their own identity and personal characteristics, including their beliefs, qualities, and how they view themselves within the social world.
Q37: A Lewis structure of aspirin without the
Q47: Of the following molecules (O<sub>3</sub>, SCl<sub>2</sub>, SO<sub>2</sub>,
Q51: Carbon dioxide is being used as an
Q52: What type of crystal structure produces an
Q62: Identify the following statement as true or
Q77: Identify the hybridization of the three carbon
Q80: In the combustion of methane, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q87: What is the bond order of B<sub>2</sub>?<br>A)0<br>B)1<br>C)2<br>D)3<br>E)1.5
Q90: Boiling points increase in the order HCl
Q150: For the reaction 2A <font face="symbol"></font> 3B