Which statement A-D regarding the reaction described by the energy profile below is not correct? 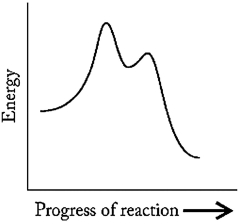
Definitions:
Indifference Curve
An indifference curve is a graph that shows a combination of two goods that give a consumer equal satisfaction and utility.
Normal Good
A good for which demand increases when income increases and falls when income decreases, all other factors being equal.
Income Increased
A rise in the amount of money earned from work, investments, or other sources.
Consumption of Strawberries
Refers to the amount of strawberries that are eaten or used by consumers within a specific period.
Q25: A chemical equilibrium <sub> </sub><sub> </sub>A <img
Q42: Phosphoric acid is a triprotic acid, ionizing
Q51: The rate of popcorn popping at different
Q68: Which of the following gases do you
Q69: Which of the following compounds contains a
Q102: Which unit cell contains the least atoms?<br>A)fcc<br>B)bcc<br>C)sc<br>D)both
Q119: In a(n) _ step of a reaction
Q119: In the following reaction, which species is
Q123: A solution is prepared by adding 0.250
Q161: Carbon monoxide has a very weak dipole