Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 H2O  H2PO4 H3O
H2PO4 H3O 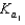 H2PO4 H2O
H2PO4 H2O  HPO42 H3O
HPO42 H3O 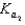
HPO42 H2O  PO43 H3O
PO43 H3O 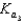 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Definitions:
Culture Bias
The tendency to interpret or judge phenomena by standards inherent to one's own culture, potentially leading to misunderstanding or prejudice.
Sampling Bias
A statistical bias that occurs when the sample chosen is not representative of the population being studied, potentially leading to distorted findings.
IQ Test
A standardized test designed to measure human intelligence as distinct from attainments, often used to assess cognitive abilities and potential.
Dissociative Identity Disorder
A psychological disorder marked by the presence of two or more distinct personality states or identities.
Q19: Because the triple point of water is
Q24: Describe the similarities and differences between a
Q26: For the following hypothetical equilibrium, what is
Q30: A concentration cell is constructed by using
Q46: Given the following reactions and associated equilibrium
Q54: Formic acid is a weak acid naturally
Q77: Would you expect the linear allotrope of
Q83: A scientist conducts an experiment to determine
Q129: Which is not true about a crystallographic
Q162: What type of motion is depicted in