According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about NO, NO, and NO? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 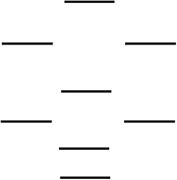
Definitions:
Unlevered Firm
A company or business that operates without the use of borrowed money or debt in its capital structure.
Unlevered Cost
The cost of an investment that has not been affected by the inclusion of debt, often referenced in the context of cost of capital.
After-Tax Net
The amount of money that remains after all taxes have been deducted from revenue or income.
Annual Interest
The interest amount that is earned or paid on a financial instrument in one calendar year.
Q49: What is the minimum energy that a
Q79: Which statement A-D about chemical bonds is
Q81: Which electron-pair geometry corresponds to a steric
Q110: A 75 mg sample of a natural
Q128: In the following Born-Haber cycle for the
Q130: Which of the following can be used
Q137: What does the line indicated by the
Q142: What wavelength of light is required to
Q153: Which is the dominant interaction that leads
Q160: Which statement regarding a <font face="symbol"></font> bond