According to the molecular orbital energy-level diagram below, which one of the following statements is not correct about NO, NO, and NO? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 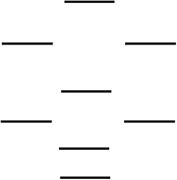
Definitions:
Marginal Cost
The increase in total cost that arises from an additional unit of production.
Average Total Cost
Average total cost is the total cost of production divided by the number of units produced, indicating the cost per unit.
Price War
Successive, competitive, and continued decreases in the prices charged by firms in an oligopolistic industry. At each stage of the price war, one firm lowers its price below its rivals’ price, hoping to increase its sales and revenues at its rivals’ expense. The war ends when the price decreases cease.
Limit Pricing
A strategy used by dominant firms to set prices low enough to discourage entry into the market by potential competitors.
Q26: Using the energy-level diagram for valence orbitals
Q57: According to de Broglie, if the circumference
Q65: Which of the following compounds has the
Q73: Which one of the ionic compounds below
Q124: Which graph best describes how the vapor
Q128: A proton in a cyclotron has a
Q129: A solution is prepared by adding 1.50
Q142: What is the molality of 153 mL
Q146: What is the hybridization of the iodine
Q147: Which of these molecules do not have