Which orbital has the lowest energy in a multielectron atom? The quantum numbers are given as (n, 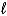 , ml) .
, ml) .
Definitions:
°C
A scale and unit of measurement for temperature where 0°C is the freezing point of water and 100°C is its boiling point at 1 atmosphere of pressure.
Atm
A unit of pressure, also known as atmosphere, approximately equal to the earth's atmospheric pressure at sea level.
Temperature
A measure of the average kinetic energy of the particles in a system, signifying how hot or cold the system is.
°C
Degrees Celsius, a unit of temperature on the Celsius scale, where 0°C represents the freezing point of water, and 100°C its boiling point at sea level.
Q30: According to Coulomb's law, which ionic compound
Q40: Arrange the first four halogens in order
Q46: Explain why the boiling point of nitrogen
Q59: How much work does a gas do
Q84: Which of the following atoms or ions
Q99: Copper forms a cation with a charge
Q99: Which is the dominant interaction between water
Q101: Determine the molecular geometry of CF<sub>2</sub>Cl<sub>2</sub>.<br>A)linear<br>B)bent<br>C)trigonal bipyramidal<br>D)tetrahedral<br>E)trigonal
Q113: Based on the positions of the atoms
Q182: Draw a structure showing the geometry of