Using the following data for water, determine the final temperature when 100 g of ice at 10 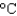 is heated with 350 kJ of energy. Boiling point
is heated with 350 kJ of energy. Boiling point
373 K
Melting point
273 K
Enthalpy of vaporization
2,260 J/g
Enthalpy of fusion
334 J/g
Specific heat capacity (solid)
2) 11 J/(g K)
Specific heat capacity (liquid)
4) 18 J/(g K)
Specific heat capacity (gas)
2) 08 J/(g K)
Definitions:
Financial Projections
Estimates of the future financial performance of a company, including income, expenses, and net profit.
Top-Down Planning
A planning approach where decision-making starts at the highest level and moves down through the hierarchy of an organization.
Projected Financial Statements
Financial documents that predict a company’s future financial performance based on current trends, assumptions, and expected future events.
Scenario Analyses
A process of examining and evaluating possible events or scenarios the future could hold and their potential impacts on business operations.
Q4: Which of these statements correctly describes why
Q7: In an experiment, 7.5 mol of a
Q11: If a chemical reaction causes the temperature
Q57: Aqua regia is a mixture of hydrochloric
Q106: The Rutherford-Geiger-Marsden gold foil experiments paved the
Q128: The combustion of heptane (C<sub>7</sub>H<sub>16</sub>) forms carbon
Q130: In comparing the dark Fraunhofer lines with
Q138: State the first law of thermodynamics. Describe
Q138: In a chemical reaction, bonds are broken
Q144: Nitrite (NO<sub>2</sub><font face="symbol"><sup></sup></font>) is an important nutrient