At a certain elevation, the boiling point of water is 98.5 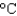 . How much energy is needed to heat 35.0 mL of water to the boiling point at this elevation if the water initially was at 23.4C? (CP 75.38 J/(mol ·
. How much energy is needed to heat 35.0 mL of water to the boiling point at this elevation if the water initially was at 23.4C? (CP 75.38 J/(mol · 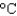 ) , d 1.00 g/mL)
) , d 1.00 g/mL)
Definitions:
Adolescents
Young people in the transitional stage of physical and psychological development from childhood to adulthood, typically aged between 13 and 19.
School Transitions
The process of moving from one educational stage or environment to another, which can include changes in school, grade, or education levels.
Greater Risks
The increased likelihood of encountering negative outcomes or dangers.
Adolescents
Individuals in the developmental stage between childhood and adulthood, typically aged 10 to 19, experiencing rapid physical, emotional, and social changes.
Q7: In a demonstration of strong electrolytes, weak
Q25: At Sal's Sandwich Shop, a regular club
Q33: Dialuminum hexachloride, Al<sub>2</sub>Cl<sub>6</sub> (266.66 g/mol), is an
Q42: Indicate which of the following photons can
Q47: If 100 mL of 3.0 M solution
Q52: According to Coulomb's law, which ionic compound
Q67: Do you expect the nitrogen-oxygen bond length
Q102: A sample of gas at 30<font face="symbol"></font>C
Q112: In carrying out a titration of a
Q151: The electron configuration of a manganese ion