Given the following thermochemical equation detailing the combustion of methane CH4(g) 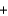 2O2(g)
2O2(g)  CO2(g)
CO2(g) 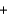 2H2O(g) Hrxn 802kJ/mol CH4
2H2O(g) Hrxn 802kJ/mol CH4
Determine the amount of energy released when 25.0 g of methane undergoes combustion.
Definitions:
Developing Nations
Countries with a lower living standard, underdeveloped industrial base, and low Human Development Index relative to other countries.
Wealthy
Having a substantial amount of money, resources, or assets; rich.
Poverty Threshold
The lowest amount of money required to maintain a satisfactory quality of life in a specific country.
Household Size
The number of individuals living in a single domestic unit, whether related by blood, marriage, adoption, or cohabitation.
Q7: Which of the following fuels has the
Q38: Which of the following atoms can have
Q42: Give an example of an ionic compound.
Q46: Identify which of the following bonds is
Q56: What is the formal charge on the
Q64: The best definition of the enthalpy change
Q82: Which statement A-D about the relationship between
Q111: One mole is defined as _<br>A)the number
Q119: Who was the first scientist to determine
Q125: Which one of the following statements is