The integrated circuits in your cell phone and computer are made from the semiconductor silicon. The silicon is obtained from a really inexpensive starting material, sand, which is primarily SiO2. One step in the purification of silicon is to separate it from solid impurities by forming the gas silicon tetrachloride. Given the following reactions, what is the overall enthalpy change in converting 1.00 mol of silicon dioxide into pure silicon? ReactionH (kJ)
SiO2(s) 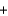 2C(s)
2C(s)  Si(impure s)
Si(impure s) 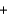 2CO(g) 690
2CO(g) 690
Si(impure s) 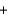 2Cl2(g)
2Cl2(g)  SiCl4(g) 657
SiCl4(g) 657
SiCl4(g) 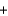 2Mg(s)
2Mg(s)  2MgCl2(s)
2MgCl2(s) 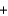 Si(s) 625
Si(s) 625
Definitions:
Brand Purpose
The reason a brand exists beyond making a profit, often framed as its contribution to society or consumers' lives, enhancing its emotional connection with customers.
Brand Storytelling
The use of narrative and creative expression to communicate a brand’s values, history, and mission to engage consumers emotionally.
Skilled Craftsperson
An individual with specialized training and high expertise in a particular craft or trade.
Q2: How many mL of hexadecane (C<sub>16</sub>H<sub>34</sub>) are
Q12: In one analysis, 3.01 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q25: Explain why the constants a and b
Q31: Which sequence does not correctly arrange the
Q38: A reaction that takes place between dissolved
Q64: Which one of the following is the
Q95: The molar mass of a gas is
Q104: Which substance listed below contains the most
Q105: What is the formula mass of (NH<sub>4</sub>)<sub>3</sub>PO<sub>4</sub>
Q161: In a mixture of hydrogen and helium