Multiple Choice
Use the following information to determine the enthalpy for the reaction shown below. S(s) O2(g) SO2(g) H ?
S(s) 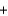
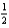 O2(g)
O2(g)  SO3(g)
SO3(g) 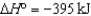 2SO2(g)
2SO2(g) 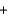 O2(g)
O2(g)  2SO3(g)
2SO3(g) 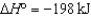
Definitions:
Related Questions
Q5: What is the formal charge of each
Q13: Give an example of a nonmetal.
Q18: Which orbital does not fill before a
Q27: Give the names of the three quantum
Q35: Which statement about isotopes of the same
Q44: In the following reaction, which element is
Q67: Which of the following compounds are soluble
Q69: Use the following information to determine the
Q87: Place these types of radiation in order
Q147: A light detector based on the photoelectric