Determine the standard enthalpy of formation for NO given the following information about the formation of NO2 under standard conditions, and 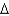
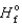 (NO2)
(NO2) 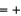 33.2 kJ/mol. 2NO(g)
33.2 kJ/mol. 2NO(g) 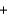 O2(g)
O2(g)  2NO2(g) Hrxn
2NO2(g) Hrxn  114.2 kJ
114.2 kJ
Definitions:
Ledger Accounts
Record-keeping systems that track financial transactions through debits and credits to maintain the financial health and status of an entity.
Financial Statements
Financial statements are formal records of the financial activities and position of a business, including the balance sheet, income statement, and cash flow statement.
Accruals
Accounting method that records revenues and expenses when they are incurred, regardless of when cash transactions occur.
Unrecorded Expense
A cost or liability that has been incurred but not yet documented or included in the financial records or statements of a company.
Q15: Identify which of the following compounds are
Q44: How many gold atoms are there in
Q50: A diver jumps off a diving board.
Q74: What are the principal and angular momentum
Q75: Oxygen is best described as a _<br>A)metalloid.<br>B)metal.<br>C)transition
Q83: Draw two reasonable Lewis structures for sulfurous
Q98: Which statement about electromagnetic radiation is not
Q107: What m<sub>l</sub> quantum numbers are possible when
Q118: Which of the following is an alkaline
Q123: Use the following information to determine the