Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction.
2N2H4(g) 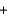 2NO2(g)
2NO2(g)  3N2(g)
3N2(g) 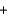 4H2O(g) Hrxn ? kJ
4H2O(g) Hrxn ? kJ
SubstanceH  in kJ/mol
in kJ/mol
N2H4(g)95.4
NO2(g)33.1
H2O(g)241.8
Definitions:
Stress-Reduction Techniques
Methods or practices designed to decrease a person's level of stress and improve their ability to cope with stressors.
Partial Hospitalization Program
A structured program of outpatient psychiatric services as an alternative to inpatient psychiatric care, providing intensive therapy and treatment.
Prophylactic Double Mastectomy
A preventive surgical procedure where both breasts are removed to significantly reduce the risk of breast cancer.
Extramarital Affair
A sexual relationship or a romantic friendship or passionate attachment between two people, where at least one individual is married to someone else.
Q30: A mixture of cyclopropane gas (C<sub>3</sub>H<sub>6</sub>) and
Q30: Which arrangement is in the correct order
Q32: Ammonia (NH<sub>3</sub>) is a weak base that
Q62: Dry air consists of 78.1% nitrogen (28
Q69: Which of the following metals would be
Q72: Motor fuels are mixtures of hydrocarbons, but
Q93: Which one of the following statements regarding
Q115: You have a summer job in a
Q145: The amount of blood in the human
Q156: The energy of a one-electron atom is