At a certain elevation, the boiling point of water is 98.5 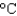 . How much energy is needed to heat 35.0 mL of water to the boiling point at this elevation if the water initially was at 23.4C? (CP 75.38 J/(mol ·
. How much energy is needed to heat 35.0 mL of water to the boiling point at this elevation if the water initially was at 23.4C? (CP 75.38 J/(mol · 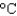 ) , d 1.00 g/mL)
) , d 1.00 g/mL)
Definitions:
Cohabitate
The act of living together and sharing a living space by non-married individuals, often in a romantic relationship.
Breakup Rate
The frequency or likelihood of relationships ending over a specified period, often used in the context of romantic partnerships.
Same-Sex Couples
Relationships involving two individuals of the same sex, either legally recognized as a marriage or akin to marriage.
Heterosexual Couples
Pairs of individuals of the opposite sex who are in a romantic and/or sexual relationship with each other.
Q1: Which orbital has the lowest energy in
Q28: Rank the following species in order of
Q46: Identify the base in the following acid-base
Q64: What is the speed of an argon
Q82: Silver salts are used in black-and-white photography.
Q118: Which of the following is an alkaline
Q118: What is the difference in the chemistry
Q120: The combustion of fossil fuels is the
Q126: A hot air balloon, under the conditions
Q129: If the molar concentration of sodium sulfate