The integrated circuits in your cell phone and computer are made from the semiconductor silicon. The silicon is obtained from a really inexpensive starting material, sand, which is primarily SiO2. One step in the purification of silicon is to separate it from solid impurities by forming the gas silicon tetrachloride. Given the following reactions, what is the overall enthalpy change in converting 1.00 mol of silicon dioxide into pure silicon? ReactionH (kJ)
SiO2(s) 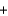 2C(s)
2C(s)  Si(impure s)
Si(impure s) 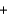 2CO(g) 690
2CO(g) 690
Si(impure s) 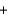 2Cl2(g)
2Cl2(g)  SiCl4(g) 657
SiCl4(g) 657
SiCl4(g) 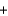 2Mg(s)
2Mg(s)  2MgCl2(s)
2MgCl2(s)  Si(s) 625
Si(s) 625
Definitions:
HIV Transmission
The spread of the Human Immunodeficiency Virus, which can occur through bodily fluids, including blood, semen, vaginal secretions, and breast milk.
Male-female Intercourse
A biological process involving a sexual act between a male and a female, often resulting in reproduction.
Emerging Adulthood
A phase of the life span between adolescence and full-fledged adulthood, typically ranging from late teens through the twenties.
Young Adulthood
A life stage characterized by the transition into full independence, exploring personal and financial milestones, typically ranging from late teens to mid-20s.
Q7: Arizona was the site of a 400,000-acre
Q20: Nitrous oxide (N<sub>2</sub>O, also known as laughing
Q28: The average car emits 8.0 kg
Q38: Which of the following atoms can have
Q48: The SI unit of force is the
Q80: Hydrogen peroxide decomposes to produce water
Q112: In carrying out a titration of a
Q119: The pressure gauge on a 100-L cylinder
Q124: What is the chemical formula for potassium
Q129: Which of the following is quantized?<br>A)the trajectory