Determine the enthalpy of formation for glucose (C6H12O6) given the following enthalpies of formation (CO2(g) , 393.5 kJ/mol; H2O(l) , 285.8 kJ/mol) and the enthalpy for the reaction shown below. C6H12O6(s) 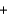 6O2 (g)
6O2 (g)  6CO2(g)
6CO2(g) 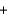 6H2O(l) H
6H2O(l) H  2801 kJ
2801 kJ
Definitions:
Invoice Description
A detailed statement showing goods sold or services provided, their prices, and the total amount to be paid.
System Recording
The process of capturing and documenting operations or events within a system for review or analysis purposes.
Financial Transactions
Deals with the process of exchanging assets such as money between two or more parties.
Itemization
Refers to the process of breaking down a list or group into individual elements for detailed analysis or reporting.
Q1: A 5 L diving tank is filled
Q44: Which polyatomic ion is not labeled correctly?<br>A)NH<sub>4</sub><sup>+</sup>ammonium<br>B)ClO<sub>4</sub><sup>-</sup>
Q71: Describe the difference between potential energy and
Q87: Which carbon-halogen bond is the most polar?
Q93: If a mass of 100.0 g moves
Q98: Which statement about electromagnetic radiation is not
Q112: What is the pressure in the gas
Q123: Use the following information to determine the
Q135: What is the molar mass of glucose,
Q148: The cold inflation pressure of many car